SceneRay Co Ltd, a medical devices company in SIP, recently received the registration approval from the National Medical Products Administration of China for its deep brain stimulation (DBS) system which is composed of an implementable neurostimulator, a lead and an extension. This system is pioneering invasive brain-computer interface (BCI) device worldwide for treating drug addiction.
SceneRay focuses on development of deep brain stimulation solutions which can provide relief to patients with Parkinson’s disease and other movement and psychiatric disorders.
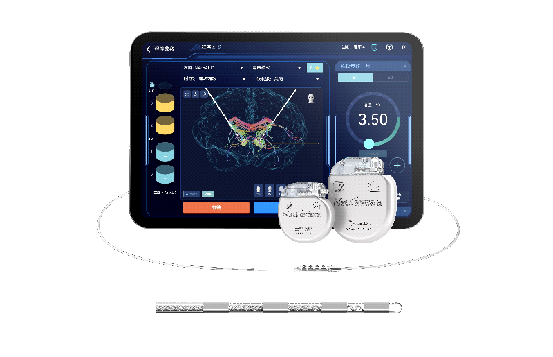
DBS is a neurosurgical procedure that involves implanting electrodes in the brain to deliver electrical impulses, helping to treat various neurological and psychiatric conditions.
The DBS system developed by SceneRay boasts a dual-target stimulation on the nucleus accumbens and anterior limb of the internal capsule, thereby aiding in preventing relapse in patients with severe, treatment-resistant opioid use disorder.

It is worth mentioning that this DBS technology has also secured the certification of the US Food and Drug Administration (FDA).
December 17, 2025







