On Dec 18, a major milestone in Chinese medical innovation was achieved as two Phase III clinical study outcomes for the mazdutide achieved by SIP-headquartered Innovent Biologics in collaboration with several top Chinese endocrinology and metabolism experts and leading R&D organizations were published in the prestigious international science journal Nature.
This marks the first time Nature has published the results of two associated Phase III studies in the field of endocrinology and metabolism. Furthermore, mazdutide is now the world’s only GLP-1 receptor agonist to achieve publications in both Nature and The New England Journal of Medicine. This has highlighted China’s significant progress in original innovation and clinical research for metabolic diseases.


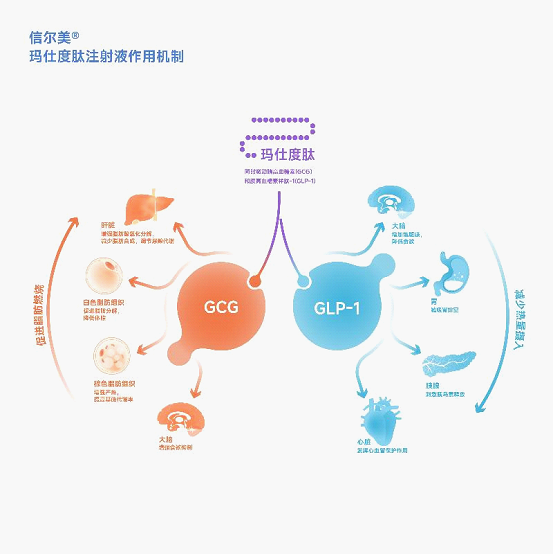
The published clinical study results are all based on data from Chinese participants, demonstrating the drug’s superior efficacy in blood sugar management and weight reduction compared to placebo or dulaglutide. The drug also showed benefits for cardiometabolic, liver and kidney health, with a favorable safety profile.
Currently, the drug has been approved in China for both diabetes and weight management, and a Phase III study for adolescent obesity, the first of its kind in China, is set to launch soon. Moreover, the National Medical Products Administration of China is reviewing a marketing application for a 9mg dose for adult weight control.
December 18, 2025







